Serum Osmolality Test: What Is It & Why Is It Done?
What if a simple blood test could unlock critical insights into your overall health and well-being? A serum osmolality test, also known as a blood osmolality test, is a powerful diagnostic tool that can unveil hidden imbalances within your body, potentially preventing serious health complications.
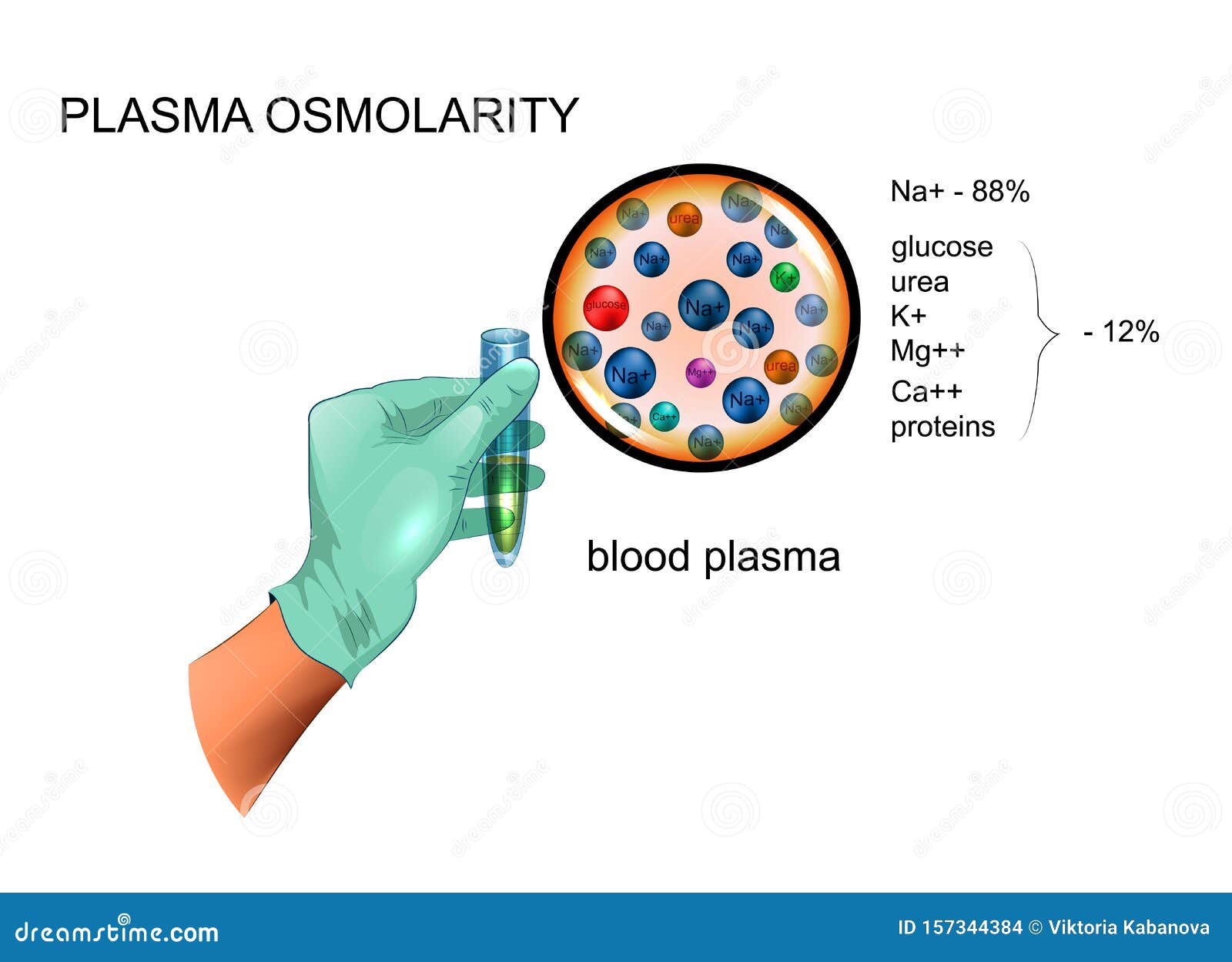
This seemingly simple test is a window into the intricate world of your body's fluid and electrolyte balance. Its a crucial diagnostic assessment that measures the concentration of particleselectrolytes, glucose, and ureain your bloodstream. This measurement, expressed as osmolality, provides essential clues about hydration status, kidney function, and the presence of any underlying conditions.
Understanding the role of osmolality is akin to understanding the flow of a river. It assesses the concentration of dissolved particles within a fluid, such as blood, urine, or even stool. These particles include crucial elements like sodium, potassium, chloride, glucose, and urea. By evaluating these components, healthcare professionals can gain deep insights into your body's water balance and the efficient operation of your kidneys.
In essence, serum osmolality measures the concentration of solutes within the liquid portion of your blood, known as serum. Serum osmolality determines the osmolar concentration of plasma and is a critical indicator of your body's internal environment. Think of it as a snapshot of your internal chemistry.
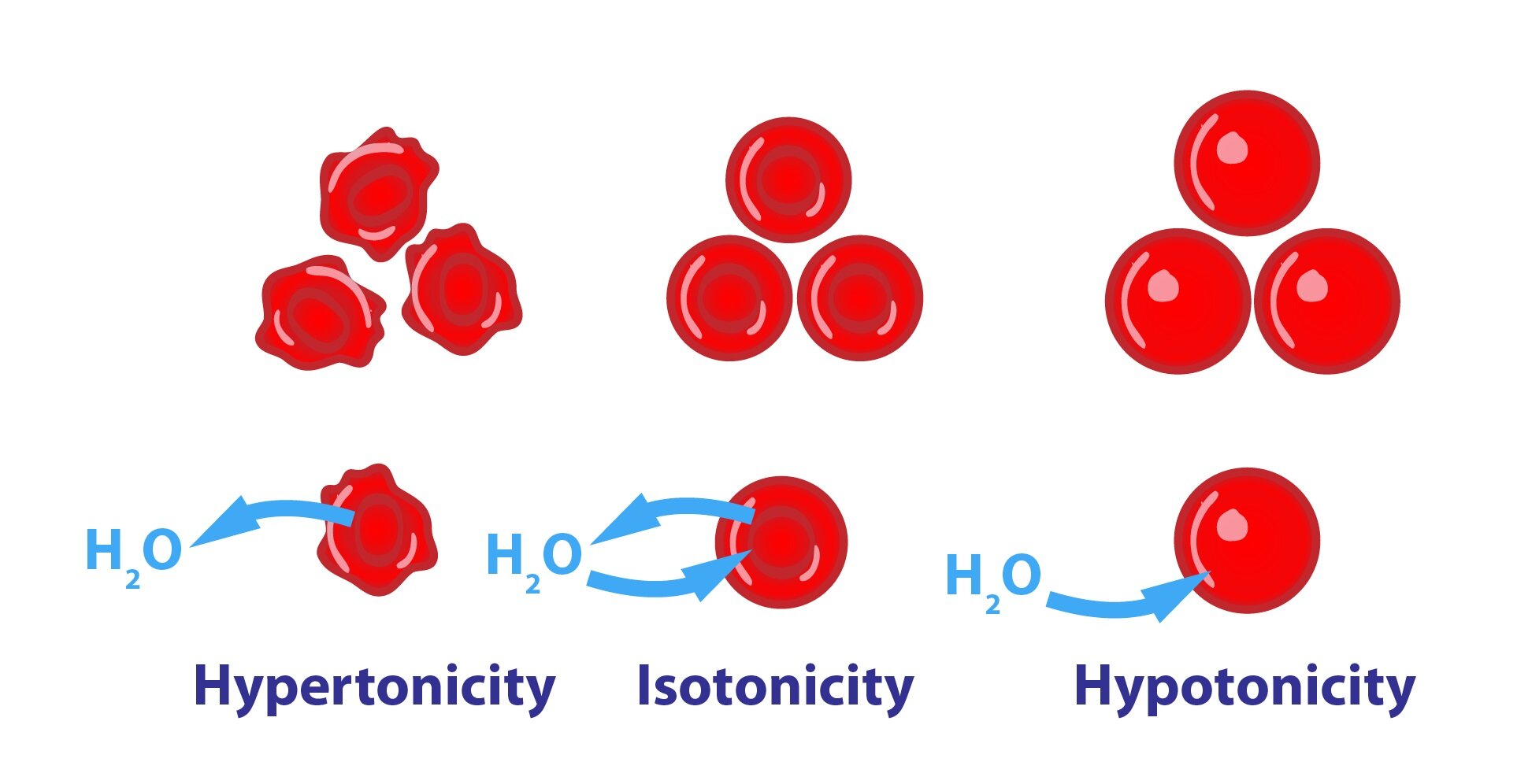
The test works on a basic principle: water will always flow from areas with lower osmolality to those with higher osmolality, a phenomenon known as osmosis, so long as a separating membrane allows the movement. In a healthy body, the kidneys play a crucial role in maintaining this delicate balance. They modulate the electrolyte balance of plasma through intricate filtration mechanisms, responding to signals like thirst and restoring lost fluids.
Several factors can influence serum osmolality levels. Elevated levels may stem from conditions like hypernatremia (high sodium), dehydration, hyperglycemia (high blood sugar), or the ingestion of substances such as ethanol, methanol, or ethylene glycol. Conversely, low serum osmolality could be a sign of overhydration or hyponatremia (low sodium).
The test itself involves a straightforward procedure. A healthcare professional will draw a blood sample from a vein, typically in your arm. This small sample is then analyzed in a laboratory to measure the concentration of various substances. The process itself is relatively quick and may only cause a slight sting upon needle insertion.
| Parameter | Details |
|---|---|
| Test Name | Serum Osmolality, Blood Osmolality, Plasma Osmolality |
| What it Measures | Concentration of dissolved particles (solutes) in the blood, including electrolytes, glucose, and urea. |
| Purpose | To assess fluid and electrolyte balance, diagnose dehydration, evaluate kidney function, and screen for alcohol intoxication (using the osmolal gap). |
| Normal Range | Generally 275 to 295 mOsm/kg (varies slightly between labs and methods). |
| High Osmolality Causes | Dehydration, hypernatremia (high sodium), hyperglycemia (high blood sugar), kidney problems, ingestion of certain substances (e.g., alcohol). |
| Low Osmolality Causes | Overhydration, hyponatremia (low sodium), certain hormonal imbalances. |
| The Osmolar Gap | The difference between measured and calculated osmolality; a significant gap can indicate the presence of substances not normally found in the blood (e.g., alcohol). |
| Sample Type | Blood Serum or Plasma (often heparinized plasma) |
| Preparation | Generally, no special preparation is needed, but follow any specific instructions from your healthcare provider. |
| Reference | National Center for Biotechnology Information (NCBI) |
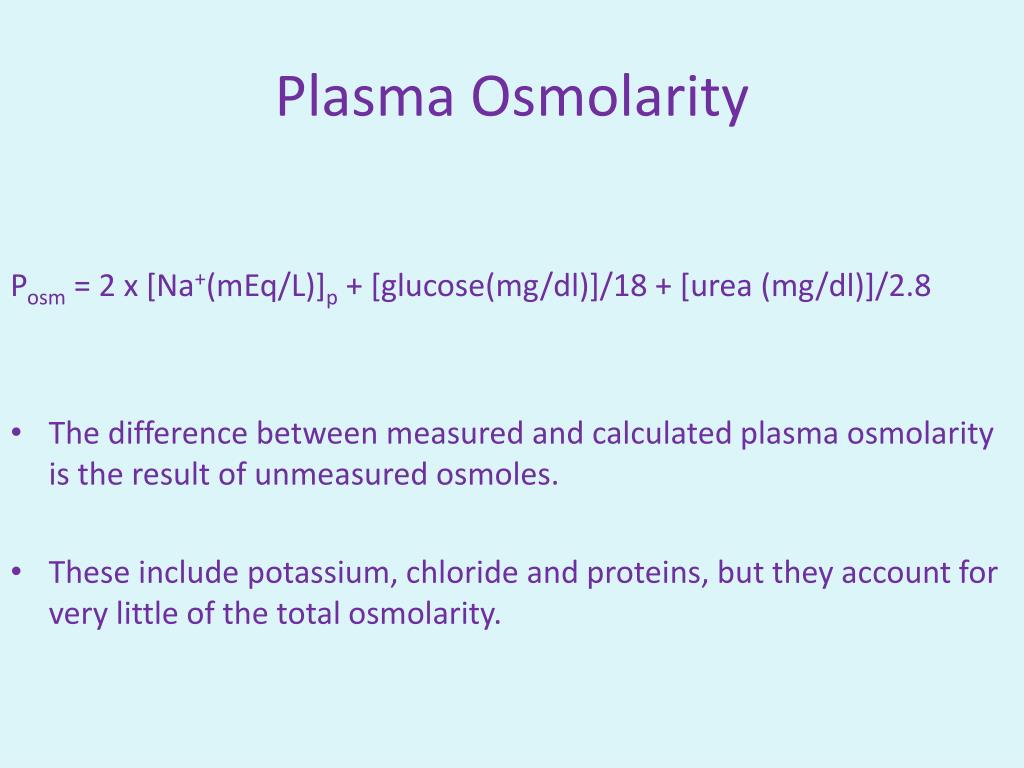
The test is particularly useful when diagnosing conditions related to fluid and electrolyte imbalances. Healthcare providers often use serum osmolality to evaluate the underlying cause of hyponatremia and can screen for alcohol intoxication using the osmolal gap. This is the difference between the measured osmolality and the osmolality calculated based on the measured concentrations of the major solutes (sodium, glucose, and urea).
In practice, the serum (or plasma) osmolality is determined by the concentrations of different solutes in the plasma, typically in millimoles per liter (mmol/L). The primary contributors to osmolality are sodium salts (mainly chloride and bicarbonate), glucose, and urea. Therefore, a calculation is often performed to determine the expected osmolality based on these major components and comparing it to the measured osmolality.
The difference between the measured and calculated osmolality is known as the osmolar gap (OG). A large positive osmolar gap, generally greater than 15, can indicate the presence of substances like ethanol or methanol, which are not typically present in significant quantities. This can be vital in toxicological assessments.
The osmolality test plays a crucial role in understanding and managing a variety of health conditions. High serum osmolality can be indicative of dehydration, high blood sugar, or even the effects of certain medications or substance ingestion. Conversely, low serum osmolality may point to overhydration or an imbalance in electrolyte levels.
In response to fluctuating osmolality levels, the body employs a sophisticated regulatory system. Osmoreceptors in the hypothalamus, which are extremely sensitive, detect even slight changes in osmolality. When the blood osmolality increases, the body releases antidiuretic hormone (ADH), also known as vasopressin. This hormone triggers the kidneys to reabsorb water, resulting in more concentrated urine and diluting the blood. Conversely, when osmolality is low, the release of ADH is suppressed, leading to less water reabsorption and more concentrated plasma.
Understanding the reference ranges for serum osmolality is key. While these ranges may vary slightly between laboratories, a common reference range falls between 275 and 295 milliosmoles per kilogram (mOsm/kg). Deviations from this range require evaluation to determine the underlying cause and guide appropriate medical intervention. For example, values higher than 295 mOsm/kg might suggest dehydration or elevated levels of certain substances, while values below 275 mOsm/kg could be related to overhydration or electrolyte imbalances.
In summary, the serum osmolality test is a valuable diagnostic tool offering insights into a patient's health, fluid balance, and the function of their kidneys, as well as providing information that can be vital in toxicology cases. Its ability to reveal potential health problems underscores its importance in clinical practice and patient care. Always consult with a healthcare professional to interpret the results of your osmolality test and to determine the appropriate course of action.